CLINICAL TRIAL DEPOT SERVICES
Reliable partner for Clinical Trial Depot Services in UAE and Egypt, serving EMEA and APAC
Trusted Partner
EPx Logistics is a trusted partner to some of the world’s leading companies in Pharma, Diagnostics, Medical Devices, E-commerce, Retail and Consumer Goods. Our list of customers in the Clinical Trial area include:

EPx Logistics offers a fully integrated solution for the management of Clinical Trials. The Clinical Trial Logistics services offered by EPx are very unique in the region. They capitalize on the already existing infrastructure of EPx (warehouses, transport vehicles), by a fully dedicated CT team of highly trained pharmacists that are responsible for its day-to-day operations. A dedicated CT team is needed in order to maintain the expertise required to meet the requirements for Clinical Trials, that are very stringent, both from a regulatory standpoint as well as from a product handling standpoint.
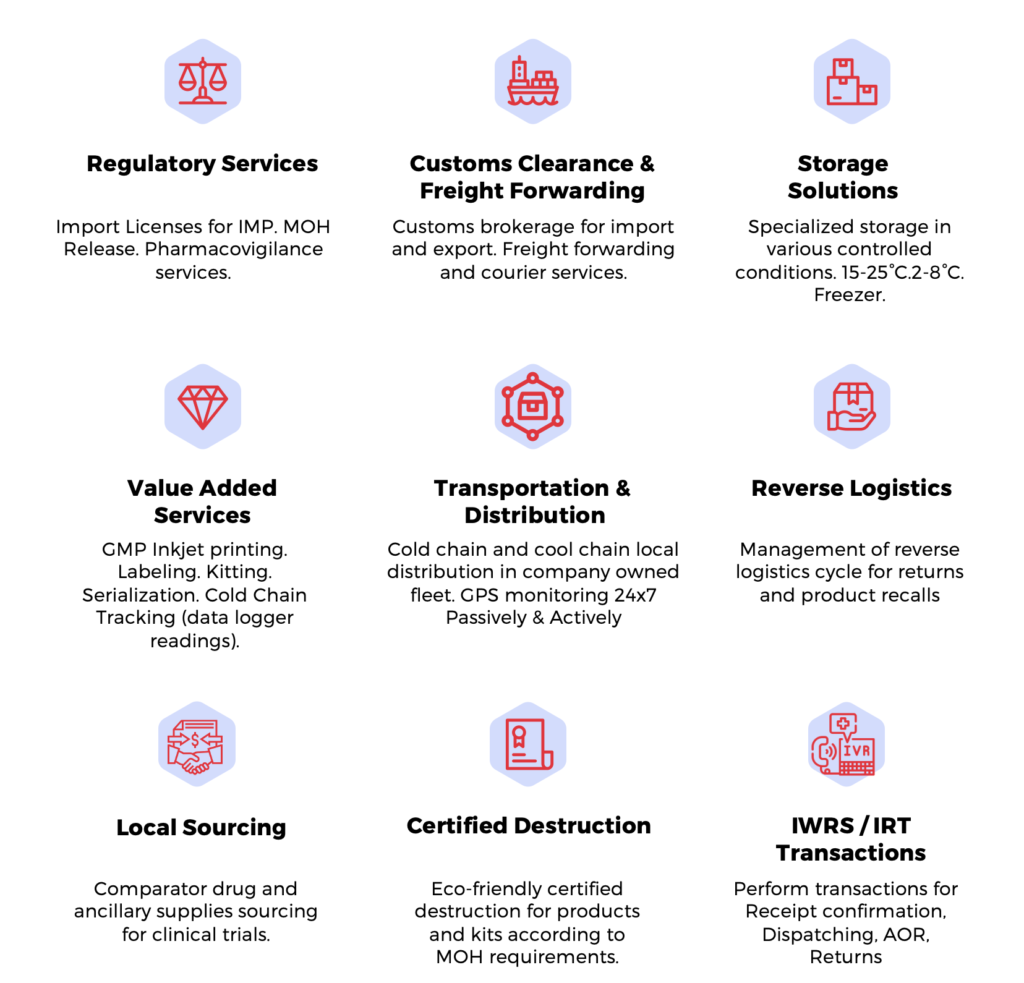
We understand how challenging the management of clinical supply can be. International trials require facilities in strategic regions combined with local insight and experience of the various regulatory requirements for investigational products to be supplied to research centers effectively and efficiently.
Our regional depots receive bulk supply shipments from global depots, sponsors and suppliers thus providing the capabilities to supply and service all research centers within that region. This allows us to serve all the key market needs for your clinical trials.
Through continuous KPI reporting, training and evaluation of performance, EPx’s dedicated CT depot team, depot manager and team leaders actively monitor the operational performance of the studies to ensure you are benefiting from the best possible solution.
The team manage and control all data and inventory through a single integrated central system, acting as the central contact point for our network and perform regular visits to maintain strong working relationships across the network. The depots are audited by EPx and by our principals annually. All depot operations are managed in accordance with Master Service Agreements (quality agreements / technical agreements) with our principals and all individual study requirements are defined in protocol-specific instruction work orders.
Strategically Located
EPx depots are strategically located to offer ideal gateways to the Middle East and unrivalled connectivity to Europe, the Indian Subcontinent, the Far East and Africa.
ABOUT OUR COMPANY

EPx Logistics and EPx Transport are the leading specialized logistics services provider in Egypt, offering specialized solutions for Health Care and other industries that require specialized storage and transport solutions. EPx Logistics and EPx Transport are members of the Egyptian Pharmex Group. Egyptian Pharmex has been a leader in the pharmaceutical services industry in Egypt since 1995.
Egypt Address:
Tagamo3at Industrial Park
Cairo-Ismailia Desert Rd
10th of Ramadan, Egypt
Phone : (+202) 44872362
Email : info@epxlogistics.com
UAE Address:
Dubai Airport Free Zone DAFZA
A08 – A11, Dubai, United Arab
Emirates
Phone : (+971) 42993055
Email : info@epxlogistics.com
